Background: Gilteritinib is a FLT3 inhibitor with demonstrated efficacy and safety in patients with FLT3-mutated relapsed or refractory (R/R) AML. The efficacy of gilteritinib in patients with prior tyrosine kinase inhibitor (TKI) therapy is not clearly defined. The phase 1/2 CHRYSALIS trial demonstrated the safety and antileukemic activity of gilteritinib in a FLT3-mutation-enriched R/R AML population (Perl AE, et al. Lancet Oncol. 2017). The phase 3 ADMIRAL trial demonstrated the superiority of gilteritinib to salvage chemotherapy (SC) in FLT3-mutated patients based on longer median overall survival (OS) with gilteritinib (9.3 vs 5.6 months; hazard ratio [HR]=0.64 [95% CI: 0.49, 0.83]; P<0.001) (Perl AE, et al. N Engl J Med. 2019). We sought to determine whether prior TKI therapy affected response and survival in these two trials.
Methods: We retrospectively analyzed clinical outcomes in patients with R/R AML previously treated with TKIs midostaurin or sorafenib, before receiving 120- or 200-mg gilteritinib in the CHRYSALIS trial, or before receiving 120-mg gilteritinib in the ADMIRAL trial. Patients randomized to SC in the ADMIRAL trial were also assessed. Patients in the CHRYSALIS trial had received at least one line of prior AML therapy; patients in the ADMIRAL trial received only one line of prior AML therapy.
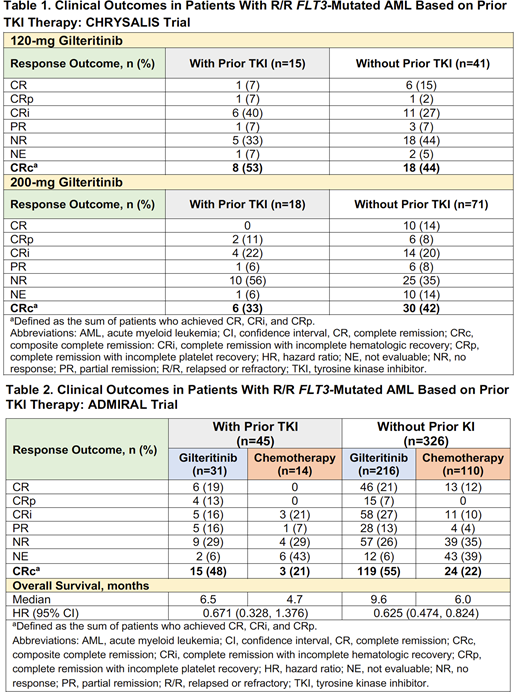
Results: Of the 145 FLT3-mutation-enriched patients who received 120- or 200-mg gilteritinib in the CHRYSALIS trial, 33 (23%; 120 mg, n=15; 200 mg, n=18) had received a prior TKI (all received sorafenib). Baseline characteristics among patients who received (n=33) or did not receive prior TKIs (n=112) were similar; median age was 56 and 61 years, respectively. Across both dose groups, FLT3 mutation types in prior TKI-treated and non-treated patients were: FLT3-ITD (88% vs 84%, respectively), FLT3-TKD (0 vs 8%, respectively), FLT3-ITD and -TKD (12% vs 6%, respectively), and unknown or missing (0 vs 2%, respectively). Rates of composite complete remission (CRc) were similar in patients who received prior TKIs (42%; n=14/33) and in those who did not (43%; n=48/112). Among patients who received prior TKIs, rates of CRc were 53% (n=8/15) in the 120-mg dose group and 33% (n=6/18) in the 200-mg dose group (Table 1); rates of CRc in patients who did not receive prior TKIs were similar across both the 120- and 200-mg dose groups (44% [n=18/41] and 42% [n=30/71], respectively). Among patients treated with prior TKIs across the 120- or 200-mg dose groups (n=33), most (73%; n=24) had received ≥3 lines of any prior AML therapy.
In the phase 3 ADMIRAL trial, 31 of 247 (13%) R/R FLT3-mutated AML patients in the gilteritinib arm and 14 of 124 (11%) patients in the SC arm had received prior TKIs. Demographic and baseline characteristics were well balanced between treatment arms and were also similar between prior TKI-treated (n=45) and non-treated patients (n=326); median age was 57 and 62 years, respectively. Among prior TKI-treated and non-treated patients, FLT3 mutation types in gilteritinib and SC arms were: FLT3-ITD (71% vs 93% and 89% vs 91%, respectively), FLT3-TKD (16% vs 7% and 7% vs 8%, respectively), and FLT3-ITD and -TKD (13% vs 0 and 1% vs 0, respectively). FLT3 mutation type was unconfirmed in 5 of 326 (2%) patients who did not receive prior TKIs (gilteritinib vs SC, 2% vs 1%, respectively). In the gilteritinib arm, CRc rates were comparable in patients who received (48%; n=15/31) and did not receive prior TKIs (55%; n=119/216); lower CRc rates were observed in the SC arm in both TKI-treated and non-treated groups (21% [n=3/14] and 22% [n=24/110], respectively) (Table 2). Median OS in patients treated with prior TKIs, albeit not statistically significant, remained high in patients treated with gilteritinib compared with those treated with SC (6.5 vs 4.7 months, respectively; HR=0.671 [95% CI: 0.328, 1.376]) (Table 2). In patients who did not receive prior TKIs, median OS was 9.6 months in the gilteritinib arm and 6.0 months in the SC arm (HR=0.625 [95% CI: 0.474, 0.824]).
Conclusions: Patients with R/R AML who received prior TKIs (midostaurin or sorafenib) were able to achieve remission with gilteritinib. High response rates with gilteritinib were observed in heavily pre-treated FLT3-mutation-enriched patients in the CHRYSALIS trial who received prior TKIs. Higher response rates with gilteritinib than with SC were observed in prior TKI-treated patients with FLT3 mutations in the ADMIRAL trial.
Perl:Takeda: Honoraria, Other: Travel costs for meeting; Syndax: Consultancy, Honoraria; Leukemia & Lymphoma Society, Beat AML: Consultancy; Agios: Consultancy, Honoraria, Other; FUJIFILM Pharmaceuticals USA, Inc: Research Funding; AbbVie Inc: Consultancy, Honoraria, Other, Research Funding; Astellas: Consultancy, Honoraria, Other: writing/editorial support, travel costs for meeting presentations related to study, Research Funding; Novartis: Honoraria, Other, Research Funding; Loxo Oncology Inc, a wholly owned subsidiary of Eli Lilly & Company: Consultancy, Honoraria, Other; Arog Pharmaceuticals Inc: Other: uncompensated consulting, travel costs for meetings; Actinium Pharmaceuticals Inc: Consultancy, Honoraria, Research Funding; New Link Genetics: Honoraria, Other; Bayer HealthCare Pharmaceuticals: Research Funding; FORMA Therapeutics: Consultancy, Honoraria, Other; Daiichi Sankyo: Consultancy, Honoraria, Other: Writing/editorial support, travel costs for meetings, Research Funding; Jazz: Honoraria, Other; Biomed Valley Discoveries: Research Funding. Altman:Cancer Expert Now: Consultancy; ASH: Consultancy; PeerView: Consultancy; Bristol-Myers Squibb: Consultancy; Fujifilm: Research Funding; AbbVie: Other: advisory board, Research Funding; BioSight: Other: No payment but was reimbursed for travel , Research Funding; Theradex: Other: Advisory Board; Immune Pharmaceuticals: Consultancy; Syros: Consultancy; Janssen: Consultancy; Kartos: Research Funding; Celgene: Research Funding; Boehringer Ingelheim: Research Funding; ImmunoGen: Research Funding; Amgen: Research Funding; Aprea: Research Funding; Amphivena: Research Funding; Genentech: Research Funding; Novartis: Consultancy; Kura Oncology: Other: Scientific Advisory Board - no payment accepted, Research Funding; Daiichi Sankyo: Other: Advisory Board - no payment but was reimbursed for travel; Agios: Other: advisory board, Research Funding; Glycomimetics: Other: Data safety and monitoring committee; Astellas: Other: Advisory Board, Speaker (no payment), Steering Committee (no payment), Research Funding; PrIME Oncology: Consultancy; France Foundation: Consultancy. Montesinos:Celgene, Pfizer, Abbvie: Consultancy; Pfizer, Abbvie, Daiichi Sankyo: Research Funding; Astellas, Novartis, Janssen: Speakers Bureau. Podoltsev:Blueprint Medicines: Consultancy, Honoraria; Astellas Pharma: Research Funding; AI Therapeutics: Research Funding; Samus Therapeutics: Research Funding; Novartis: Consultancy, Honoraria; Bristol-Myers Squib: Consultancy, Honoraria; Alexion: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Agios Pharmaceuticals: Consultancy, Honoraria; Daiichi Sankyo: Research Funding; Genentech: Research Funding; CTI biopharma: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Boehringer Ingelheim: Research Funding; Sunesis Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding; Incyte: Consultancy, Honoraria; Kartos Therapeutics: Research Funding; Arog Pharmaceuticals: Research Funding. Martinelli:Celgene: Consultancy, Speakers Bureau; Amgen: Consultancy; AbbVie: Consultancy, Research Funding; Janssen: Consultancy; Roche: Consultancy; Pfizer: Consultancy, Research Funding, Speakers Bureau; Daichii Sankyo: Consultancy, Research Funding; Incyte: Consultancy; Jazz: Consultancy. Smith:FujiFilm: Other: Research support, Research Funding; Abbvie: Other: Research Support, Research Funding; Revolution Medicines: Other: Research Support, Research Funding; Daiichi Sanyko: Consultancy, Honoraria; Sanofi: Honoraria; Astellas Pharma: Honoraria, Other: Research Support, Research Funding. Levis:Amgen: Honoraria; Menarini: Honoraria; FujiFilm: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria. Röllig:Abbvie, Novartis, Pfizer: Consultancy, Research Funding; Amgen, Astellas, BMS, Daiichi Sankyo, Janssen, Roche: Consultancy. Groß-Langenhoff:Astellas: Current Employment. Hasabou:Astellas Pharma: Current Employment. Lu:Astellas: Current Employment. Tiu:Astellas Pharma Global Development: Current Employment; Eli Lilly & Company: Current equity holder in publicly-traded company, Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.